|
Comprehensive Metabolic Panel
 Insights
Insights
|
|
Urea Nitrogen (BUN)
|

|
high
|
|
|
|
Low
|
Desirable 7 – 25
|
High
|
|
|
|
|
|
|
Creatinine
|

|
high
|
|
|
|
Low
|
Desirable 0.60 – 1.26
|
High
|
|
|
|
|
|
|
AST
|

|
good
|
|
|
|
Low
|
Desirable 10 – 40
|
High
|
|
|
|
|
|
|
Glucose
|

|
good
|
|
|
|
Low
|
Desirable 65 – 99
|
High
|
|
|
|
|
|
Note: Fasting reference interval
|
|
|
EGFR
|

|
good
|
|
|
|
|
BUN/Creatinine Ratio
|

|
good
|
|
|
|
Low
|
Desirable 6 – 22
|
High
|
|
|
|
|
|
|
Sodium
|

|
good
|
|
|
|
Low
|
Desirable 135 – 146
|
High
|
|
|
|
|
|
|
Potassium
|

|
good
|
|
|
|
Low
|
Desirable 3.5 – 5.3
|
High
|
|
|
|
|
|
|
Chloride
|

|
good
|
|
|
|
Low
|
Desirable 98 – 110
|
High
|
|
|
|
|
|
|
Carbon Dioxide
|

|
good
|
|
|
|
Low
|
Desirable 20 – 32
|
High
|
|
|
|
|
|
|
Calcium
|

|
good
|
|
|
|
Low
|
Desirable 8.6 – 10.3
|
High
|
|
|
|
|
|
|
Protein, Total
|

|
good
|
|
|
|
Low
|
Desirable 6.1 – 8.1
|
High
|
|
|
|
|
|
|
Albumin
|

|
good
|
|
|
|
Low
|
Desirable 3.6 – 5.1
|
High
|
|
|
|
|
|
|
Globulin
|

|
good
|
|
|
|
Low
|
Desirable 1.9 – 3.7
|
High
|
|
|
|
|
|
|
Albumin/Globulin Ratio
|

|
good
|
|
|
|
Low
|
Desirable 1.0 – 2.5
|
High
|
|
|
|
|
|
|
Bilirubin, Total
|

|
good
|
|
|
|
Low
|
Desirable 0.2 – 1.2
|
High
|
|
|
|
|
|
|
Alkaline Phosphatase
|

|
good
|
|
|
|
Low
|
Desirable 36 – 130
|
High
|
|
|
|
|
|
|
ALT
|

|
good
|
|
|
|
Low
|
Desirable 9 – 46
|
High
|
|
|
|
|
|
|
Estradiol
 Insights
Insights
|
|
Estradiol
|

|
high
|
|
|
|
Note: Reference range established on post-pubertal patient
population. No pre-pubertal reference range
established using this assay. For any patients for
whom low Estradiol levels are anticipated (e.g. males,
pre-pubertal children and hypogonadal/post-menopausal
females), the Quest Diagnostics Nichols Institute
Estradiol, Ultrasensitive, LCMSMS assay is recommended
(order code 30289).
Please note: patients being treated with the drug
fulvestrant (Faslodex(R)) have demonstrated significant
interference in immunoassay methods for estradiol
measurement. The cross reactivity could lead to falsely
elevated estradiol test results leading to an
inappropriate clinical assessment of estrogen status.
Quest Diagnostics order code 30289-Estradiol,
Ultrasensitive LC/MS/MS demonstrates negligible cross
reactivity with fulvestrant.
|
|
|
FSH
 Insights
Insights
|
|
FSH
|

|
low
|
|
|
|
Low
|
Desirable 1.6 – 8.0
|
High
|
|
|
|
|
|
|
Amylase
 Insights
Insights
|
|
Amylase
|

|
high
|
|
|
|
Low
|
Desirable 21 – 101
|
High
|
|
|
|
|
|
|
Creatine Kinase
 Insights
Insights
|
|
Creatine Kinase, Total
|

|
high
|
|
|
|
Low
|
Desirable 44 – 196
|
High
|
|
|
|
|
|
|
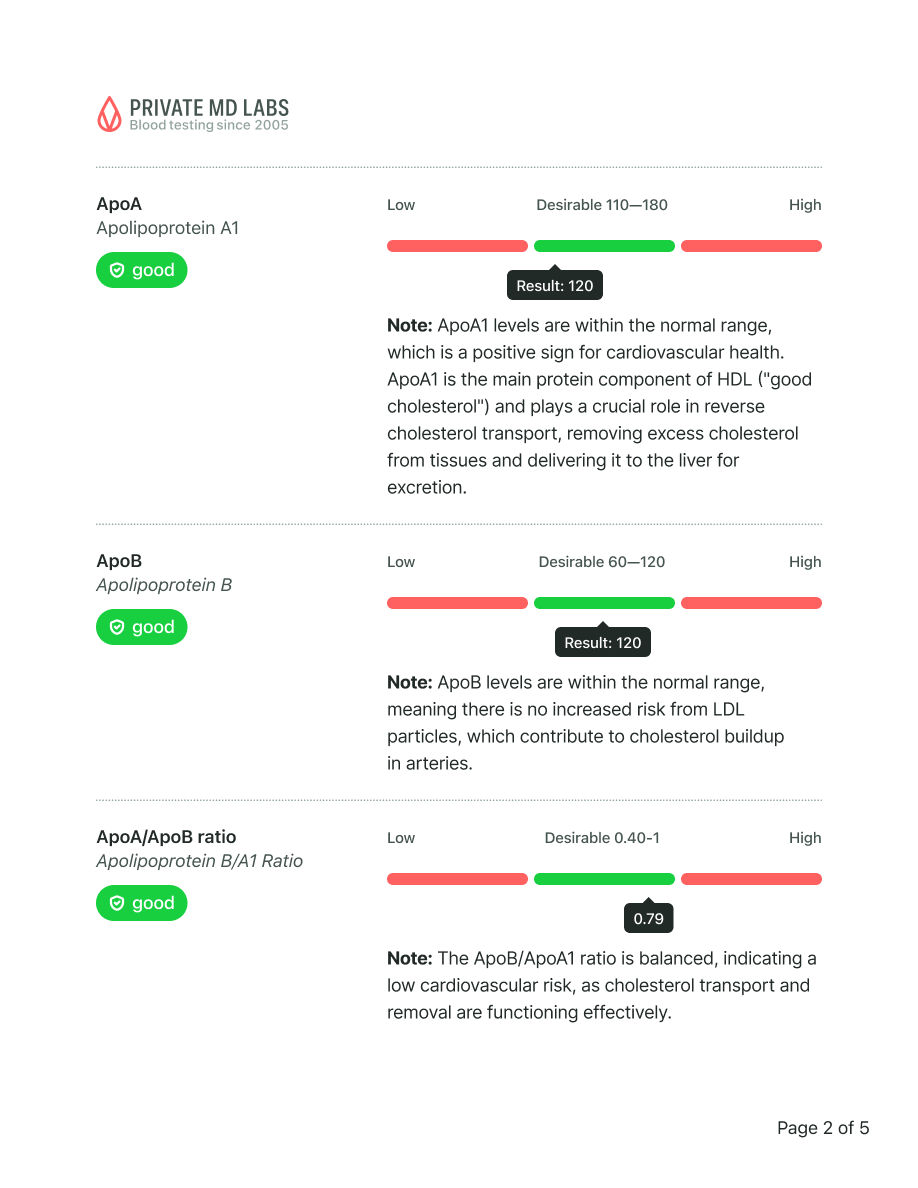
Apolipoprotein Evaluation
 Insights
Insights
|
|
Apolipoprotein B
|

|
high
|
|
|
|
Note: Reference Range: <90
Risk Category:
Optimal <90
Moderate 90-119
High > or = 120
Cardiovascular event risk category cut points (optimal,
moderate, high) are based on National Lipid Association
recommendations - Jacobson TA et al. J of Clin Lipid.
2015;9:129-169 and Jellinger PS et al. Endocr Pract.
2017;23(Suppl 2):1-87.
|
|
|
Apolipoprotein A1
|

|
good
|
|
|
|
Note: Reference Range: > or = 115
Risk Category:
Optimal > or = 115
High <115
Cardiovascular event risk category cut points (optimal,
high) are based on the AMORIS study, Walldius G et al. J
Intern Med. 2004;255:188-205.
|
|
|
Apolipoprotein B/A1 Ratio
|

|
good
|
|
|
|
Note: Reference Range: <0.77
Risk Category:
Optimal <0.77
Moderate 0.77-0.95
High >0.95
Cardiovascular event risk category cut points (optimal,
moderate, high) are based on the AMORIS study, Walldius G et
al. J Intern Med. 2004;255:188-205.
|
|
|
PTH
 Insights
Insights
|
|
Parathyroid Hormone, Intact
|

|
high
|
|
|
|
Low
|
Desirable 16 – 77
|
High
|
|
|
|
|
|
Note: Interpretive Guide Intact PTH Calcium
------------------ ---------- -------
Normal Parathyroid Normal Normal
Hypoparathyroidism Low or Low Normal Low
Hyperparathyroidism
Primary Normal or High High
Secondary High Normal or Low
Tertiary High High
Non-Parathyroid
Hypercalcemia Low or Low Normal High
|
|
|
CBC (Includes DIFF/PLT)
 Insights
Insights
|
|
White Blood Cell Count
|

|
good
|
|
|
|
Low
|
Desirable 3.8 – 10.8
|
High
|
|
|
|
|
|
|
Red Blood Cell Count
|

|
good
|
|
|
|
Low
|
Desirable 3.80 – 5.10
|
High
|
|
|
|
|
|
|
Hemoglobin
|

|
good
|
|
|
|
Low
|
Desirable 11.7 – 15.5
|
High
|
|
|
|
|
|
|
Hematocrit
|

|
good
|
|
|
|
Low
|
Desirable 35.0 – 45.0
|
High
|
|
|
|
|
|
|
MCV
|

|
good
|
|
|
|
Low
|
Desirable 80.0 – 100.0
|
High
|
|
|
|
|
|
|
MCH
|

|
good
|
|
|
|
Low
|
Desirable 27.0 – 33.0
|
High
|
|
|
|
|
|
|
MCHC
|

|
good
|
|
|
|
Low
|
Desirable 32.0 – 36.0
|
High
|
|
|
|
|
|
|
RDW
|

|
good
|
|
|
|
Low
|
Desirable 11.0 – 15.0
|
High
|
|
|
|
|
|
|
Platelet Count
|

|
good
|
|
|
|
Low
|
Desirable 140 – 400
|
High
|
|
|
|
|
|
|
MPV
|

|
good
|
|
|
|
Low
|
Desirable 7.5 – 12.5
|
High
|
|
|
|
|
|
|
Absolute Neutrophils
|

|
good
|
|
|
|
Low
|
Desirable 1500 – 7800
|
High
|
|
|
|
|
|
|
Absolute Lymphocytes
|

|
good
|
|
|
|
Low
|
Desirable 850 – 3900
|
High
|
|
|
|
|
|
|
Absolute Monocytes
|

|
good
|
|
|
|
Low
|
Desirable 200 – 950
|
High
|
|
|
|
|
|
|
Absolute Eosinophils
|

|
good
|
|
|
|
Low
|
Desirable 15 – 500
|
High
|
|
|
|
|
|
|
Absolute Basophils
|

|
good
|
|
|
|
|
Neutrophils
|

|
good
|
|
|
|
|
Lymphocytes
|

|
good
|
|
|
|
|
Monocytes
|

|
good
|
|
|
|
|
Eosinophils
|

|
good
|
|
|
|
|
Basophils
|

|
good
|
|
|
|
|
IGF 1
 Insights
Insights
|
|
IGF 1, LC/MS
|

|
good
|
|
|
|
Low
|
Desirable 83 – 456
|
High
|
|
|
|
|
|
|
Z Score (Female)
|

|
good
|
|
|
|
Note: This test was developed and its analytical performance
characteristics have been determined by Quest Diagnostics
Nichols Institute San Juan Capistrano. It has not been
cleared or approved by FDA. This assay has been validated
pursuant to the CLIA regulations and is used for clinical
purposes.
|
|
|
DHEA Sulfate
 Insights
Insights
|
|
DHEA Sulfate
|

|
good
|
|
|
|
Low
|
Desirable 93 – 415
|
High
|
|
|
|
|
|
|
Ferritin
 Insights
Insights
|
|
Ferritin
|

|
good
|
|
|
|
Low
|
Desirable 38 – 380
|
High
|
|
|
|
|
|
|
GGT
 Insights
Insights
|
|
GGT
|

|
good
|
|
|
|
Low
|
Desirable 3 – 90
|
High
|
|
|
|
|
|
|
LH
 Insights
Insights
|
|
LH
|

|
good
|
|
|
|
Low
|
Desirable 1.5 – 9.3
|
High
|
|
|
|
|
|
|
Prolactin
 Insights
Insights
|
|
Prolactin
|

|
good
|
|
|
|
Low
|
Desirable 2.0 – 18.0
|
High
|
|
|
|
|
|
|
Questassured(TM) 25 Hydroxyvitamin D(D2,D3)
 Insights
Insights
|
|
Vitamin D, 25-OH, Total
|

|
good
|
|
|
|
Low
|
Desirable 30 – 100
|
High
|
|
|
|
|
|
Note: Vitamin D, 25-Hydroxy reports concentrations of two
common forms, 25-OHD2 and 25-OHD3. 25-OHD3 indicates
both endogenous production and supplementation.
25-OHD2 is an indicator of exogenous sources such as
diet or supplementation. Therapy is based on
measurement of Total 25-OHD, with levels <20 ng/mL
indicative of Vitamin D deficiency, while levels
between 20 ng/mL and 30 ng/mL suggest insufficiency.
Optimal levels are > or = 30 ng/mL.
For additional information, please refer to
http://education.QuestDiagnostics.com/faq/FAQ199
(This link is being provided for informational/
educational purposes only.)
|
|
|
Vitamin D, 25-OH, D3
|

|
good
|
|
|
|
Note: This test was developed and its analytical performance
characteristics have been determined by Quest
Diagnostics Nichols Institute Chantilly, VA. It has
not been cleared or approved by the U.S. Food and Drug
Administration. This assay has been validated pursuant
to the CLIA regulations and is used for clinical
purposes.
|
|
|
Vitamin D, 25-OH, D2
|

|
good
|
|
|
|
Note: This test was developed and its analytical performance
characteristics have been determined by Quest
Diagnostics Nichols Institute Chantilly, VA. It has
not been cleared or approved by the U.S. Food and Drug
Administration. This assay has been validated pursuant
to the CLIA regulations and is used for clinical
purposes.
|
|
|
Sex Hormone Binding Globulin
 Insights
Insights
|
|
Sex Hormone Binding Globulin
|

|
good
|
|
|
|
Low
|
Desirable 10 – 50
|
High
|
|
|
|
|
|
|
T3
 Insights
Insights
|
|
T3, Free
|

|
good
|
|
|
|
Low
|
Desirable 2.3 – 4.2
|
High
|
|
|
|
|
|
|
T4
 Insights
Insights
|
|
T4, Free
|

|
good
|
|
|
|
Low
|
Desirable 0.8 – 1.8
|
High
|
|
|
|
|
|
|
Testosterone
 Insights
Insights
|
|
Testosterone, Total, MS
|

|
good
|
|
|
|
Low
|
Desirable 250 – 1100
|
High
|
|
|
|
|
|
Note: For additional information, please refer to
http://education.questdiagnostics.com/faq/
TotalTestosteroneLCMSMSFAQ165
(This link is being provided for informational/
educational purposes only.)
This test was developed and its analytical performance
characteristics have been determined by Quest
Diagnostics Nichols Institute Chantilly, VA. It has
not been cleared or approved by the U.S. Food and Drug
Administration. This assay has been validated pursuant
to the CLIA regulations and is used for clinical
purposes.
|
|
|
Testosterone, Free
|

|
good
|
|
|
|
Low
|
Desirable 35.0 – 155.0
|
High
|
|
|
|
|
|
Note: This test was developed and its analytical performance
characteristics have been determined by Quest
Diagnostics Nichols Institute Chantilly, VA. It has
not been cleared or approved by the U.S. Food and Drug
Administration. This assay has been validated pursuant
to the CLIA regulations and is used for clinical
purposes.
|
|
|
TSH
 Insights
Insights
|
|
TSH
|

|
good
|
|
|
|
Low
|
Desirable 0.40 – 4.50
|
High
|
|
|
|
|
|
|
Urinalysis
 Insights
Insights
|
|
Bacteria
|

|
good
|
|
|
|
|
Squamous Epithelial Cells
|

|
good
|
|
|
|
|
RBC
|

|
good
|
|
|
|
|
WBC
|

|
good
|
|
|
|
|
Leukocyte Esterase
|

|
good
|
|
|
|
|
Nitrite
|

|
good
|
|
|
|
|
Occult Blood
|

|
good
|
|
|
|
|
Ketones
|

|
good
|
|
|
|
|
Bilirubin
|

|
good
|
|
|
|
|
Glucose
|

|
good
|
|
|
|
|
PH
|

|
good
|
|
|
|
Low
|
Desirable 5.0 – 8.0
|
High
|
|
|
|
|
|
|
Specific Gravity
|

|
good
|
|
|
|
Low
|
Desirable 1.001 – 1.035
|
High
|
|
|
|
|
|
|
Appearance
|

|
good
|
|
|
|
|
Color
|

|
good
|
|
|
|
|
Protein
|

|
good
|
|
|
|
|
Hyaline CAST
|

|
good
|
|
|
|
|
Lipid Panel
 Insights
Insights
|
|
HDL Cholesterol
|

|
good
|
|
|
|
|
Triglycerides
|

|
good
|
|
|
|
|
CHOL/HDLC Ratio
|

|
good
|
|
|
|
|
Non HDL Cholesterol
|

|
good
|
|
|
|
Note: For patients with diabetes plus 1 major ASCVD risk
factor, treating to a non-HDL-C goal of <100 mg/dL
(LDL-C of <70 mg/dL) is considered a therapeutic
option.
|
|
|
Cholesterol, Total
|

|
good
|
|
|
|
|
LDL-Cholesterol
|

|
good
|
|
|
|
Note: Reference range: <100
Desirable range <100 mg/dL for primary prevention;
<70 mg/dL for patients with CHD or diabetic patients
with > or = 2 CHD risk factors.
LDL-C is now calculated using the Martin-Hopkins
calculation, which is a validated novel method providing
better accuracy than the Friedewald equation in the
estimation of LDL-C.
Martin SS et al. JAMA. 2013;310(19): 2061-2068
(http://education.QuestDiagnostics.com/faq/FAQ164)
|
|
|
Cortisol
 Insights
Insights
|
|
Cortisol, Total
|

|
good
|
|
|
|
Note: Reference Range: For 8 a.m.(7-9 a.m.) Specimen: 4.0-22.0
Reference Range: For 4 p.m.(3-5 p.m.) Specimen: 3.0-17.0
* Please interpret above results accordingly *
|
|
|
Vitamin B12
 Insights
Insights
|
|
Vitamin B12
|

|
good
|
|
|
|
Low
|
Desirable 200 – 1100
|
High
|
|
|
|
|
|
|
C-Reactive Protein
 Insights
Insights
|
|
C-Reactive Protein
|

|
good
|
|
|
|
|
Rheumatoid Factor
 Insights
Insights
|
|
Rheumatoid Factor
|

|
good
|
|
|
|
|
PSA
 Insights
Insights
|
|
PSA, Total
|

|
good
|
|
|
|
Note: The total PSA value from this assay system is
standardized against the WHO standard. The test
result will be approximately 20% lower when compared
to the equimolar-standardized total PSA (Beckman
Coulter). Comparison of serial PSA results should be
interpreted with this fact in mind.
This test was performed using the Siemens
chemiluminescent method. Values obtained from
different assay methods cannot be used
interchangeably. PSA levels, regardless of
value, should not be interpreted as absolute
evidence of the presence or absence of disease.
|
|
|
SED Rate By Modified Westergren
 Insights
Insights
|
|
SED Rate By Modified Westergren
|

|
good
|
|
|
|
|
T4 (Thyroxine)
 Insights
Insights
|
|
T4 (Thyroxine), Total
|

|
good
|
|
|
|
Low
|
Desirable 4.9 – 10.5
|
High
|
|
|
|
|
|
|
Hemoglobin A1C
 Insights
Insights
|
|
Hemoglobin A1C
|

|
good
|
|
|
|
Note: For the purpose of screening for the presence of
diabetes:
<5.7% Consistent with the absence of diabetes
5.7-6.4% Consistent with increased risk for diabetes
(prediabetes)
> or =6.5% Consistent with diabetes
This assay result is consistent with a decreased risk
of diabetes.
Currently, no consensus exists regarding use of
hemoglobin A1c for diagnosis of diabetes in children.
According to American Diabetes Association (ADA)
guidelines, hemoglobin A1c <7.0% represents optimal
control in non-pregnant diabetic patients. Different
metrics may apply to specific patient populations.
Standards of Medical Care in Diabetes(ADA).
|
|
|
Insulin
 Insights
Insights
|
|
Insulin
|

|
good
|
|
|
|
Note: Reference Range < or = 18.4
Risk:
Optimal < or = 18.4
Moderate NA
High >18.4
Adult cardiovascular event risk category
cut points (optimal, moderate, high)
are based on Insulin Reference Interval
studies performed at Quest Diagnostics
in 2022.
|
|
|
Magnesium
 Insights
Insights
|
|
Magnesium
|

|
good
|
|
|
|
Low
|
Desirable 1.5 – 2.5
|
High
|
|
|
|
|
|
|
Iron
 Insights
Insights
|
|
Iron, Total
|

|
good
|
|
|
|
Low
|
Desirable 50 – 180
|
High
|
|
|
|
|
|
|
Phosphate (AS Phosphorus)
 Insights
Insights
|
|
Phosphate (AS Phosphorus)
|

|
good
|
|
|
|
Low
|
Desirable 2.1 – 4.3
|
High
|
|
|
|
|
|
|
Prothrombin Time-INR
 Insights
Insights
|
|
PT
|

|
good
|
|
|
|
Low
|
Desirable 9.0 – 11.5
|
High
|
|
|
|
|
|
Note: For additional information, please refer to
http://education.questdiagnostics.com/faq/FAQ104
(This link is being provided for informational/
educational purposes only.)
|
|
|
INR
|

|
good
|
|
|
|
Note: Reference Range 0.9-1.1
Moderate-intensity Warfarin Therapy 2.0-3.0
Higher-intensity Warfarin Therapy 3.0-4.0
|
|
|
Folate
 Insights
Insights
|
|
Folate, Serum
|

|
good
|
|
|
|
Note: Reference Range
Low: <3.4
Borderline: 3.4-5.4
Normal: >5.4
|
|
|
Uric Acid
 Insights
Insights
|
|
Uric Acid
|

|
good
|
|
|
|
Low
|
Desirable 2.5 – 7.0
|
High
|
|
|
|
|
|
Note: Therapeutic target for gout patients: <6.0 mg/dL
|
|
|
Fibrinogen Activity
 Insights
Insights
|
|
Fibrinogen Activity, Clauss
|

|
good
|
|
|
|
Low
|
Desirable 175 – 425
|
High
|
|
|
|
|
|
|
LD
 Insights
Insights
|
|
LD
|

|
good
|
|
|
|
Low
|
Desirable 120 – 250
|
High
|
|
|
|
|
|
|
Hepatic Function Panel
 Insights
Insights
|
|
Globulin
|

|
good
|
|
|
|
Low
|
Desirable 1.9 – 3.7
|
High
|
|
|
|
|
|
|
Protein, Total
|

|
good
|
|
|
|
Low
|
Desirable 6.1 – 8.1
|
High
|
|
|
|
|
|
|
Albumin
|

|
good
|
|
|
|
Low
|
Desirable 3.6 – 5.1
|
High
|
|
|
|
|
|
|
Albumin/Globulin Ratio
|

|
good
|
|
|
|
Low
|
Desirable 1.0 – 2.5
|
High
|
|
|
|
|
|
|
Bilirubin, Total
|

|
good
|
|
|
|
Low
|
Desirable 0.2 – 1.2
|
High
|
|
|
|
|
|
|
Bilirubin, Direct
|

|
good
|
|
|
|
|
Bilirubin, Indirect
|

|
good
|
|
|
|
Low
|
Desirable 0.2 – 1.2
|
High
|
|
|
|
|
|
|
Alkaline Phosphatase
|

|
good
|
|
|
|
Low
|
Desirable 36 – 130
|
High
|
|
|
|
|
|
|
AST
|

|
good
|
|
|
|
Low
|
Desirable 10 – 40
|
High
|
|
|
|
|
|
|
ALT
|

|
good
|
|
|
|
Low
|
Desirable 9 – 46
|
High
|
|
|
|
|
|
|
Thyroglobulin Antibodies
 Insights
Insights
|
|
Thyroglobulin Antibodies
|

|
good
|
|
|
|
|
CA 125
 Insights
Insights
|
|
CA 125
|

|
good
|
|
|
|
Note: This test was performed using the Siemens
Chemiluminescent method. Values obtained from
different assay methods cannot be used
interchangeably. CA 125 levels, regardless of
value, should not be interpreted as absolute
evidence of the presence or absence of disease.
|
|